Can Atoms Be Observed Under A Microscope ?
Atoms cannot be observed directly under a traditional optical microscope due to their extremely small size. The wavelength of visible light is much larger than the size of atoms, making them beyond the resolution limit of optical microscopes. However, there are advanced techniques that allow for the indirect observation of atoms. Scanning tunneling microscopy (STM) and atomic force microscopy (AFM) are two such techniques that can image atoms. These techniques involve scanning a sharp probe over the surface of a sample and measuring the interaction between the probe and the atoms. By mapping out these interactions, it is possible to create images that reveal the positions of individual atoms. These techniques have revolutionized our understanding of atomic structures and have been instrumental in various fields of science and nanotechnology.
1、 Atomic Force Microscopy: Directly imaging atoms using a specialized microscope.
Yes, atoms can be observed under a microscope using a technique called Atomic Force Microscopy (AFM). AFM is a specialized type of microscope that allows for the direct imaging of atoms and molecules at the nanoscale level.
Unlike traditional optical microscopes, which use light to observe objects, AFM uses a tiny probe to scan the surface of a sample. This probe, typically a sharp tip attached to a cantilever, interacts with the atoms on the surface of the sample, producing a three-dimensional image of the atomic arrangement.
AFM works by measuring the forces between the probe and the atoms on the surface. As the probe scans the sample, it moves up and down in response to these forces, and these movements are detected and used to create an image. The resolution of AFM is so high that individual atoms can be observed and manipulated.
In recent years, advancements in AFM technology have further improved its capabilities. For example, high-speed AFM allows for the real-time imaging of dynamic processes, such as chemical reactions or biological events, at the atomic scale. Additionally, non-contact AFM techniques have been developed, which minimize the interaction between the probe and the sample, enabling even more precise imaging.
It is important to note that while AFM provides a powerful tool for observing atoms, it does not directly visualize the electron cloud surrounding the nucleus. Instead, it relies on the interaction between the probe and the atoms' outermost electrons. Nevertheless, AFM has revolutionized our ability to study and understand the nanoscale world, opening up new possibilities in various fields, including materials science, biology, and nanotechnology.

2、 Scanning Tunneling Microscopy: Visualizing atoms by measuring electron tunneling.
Yes, atoms can be observed under a microscope using a technique called Scanning Tunneling Microscopy (STM). STM is a powerful tool that allows scientists to visualize individual atoms by measuring the electron tunneling between a sharp metal tip and a sample surface.
In STM, a fine metal tip is brought very close to the surface of a conductive material. A small voltage is applied between the tip and the sample, creating a tunneling current. By monitoring this current, scientists can map the surface topography of the material at the atomic scale. The tip is scanned across the surface, and a computer generates an image based on the variations in tunneling current, providing a detailed representation of the atomic arrangement.
STM has revolutionized our understanding of materials and nanoscale structures. It has been used to observe individual atoms, molecules, and even the manipulation of atoms to create nanostructures. This technique has provided valuable insights into the behavior and properties of materials, leading to advancements in fields such as physics, chemistry, and materials science.
It is important to note that STM is limited to conductive materials, as the tunneling current relies on the flow of electrons. Additionally, STM requires a controlled environment, typically performed in ultra-high vacuum conditions to minimize interference from external factors.
In recent years, advancements in STM technology have allowed for even higher resolution imaging and the ability to manipulate individual atoms. Researchers have pushed the boundaries of STM to explore new frontiers in nanoscience and nanotechnology, enabling the development of novel materials and devices with unprecedented control at the atomic level.
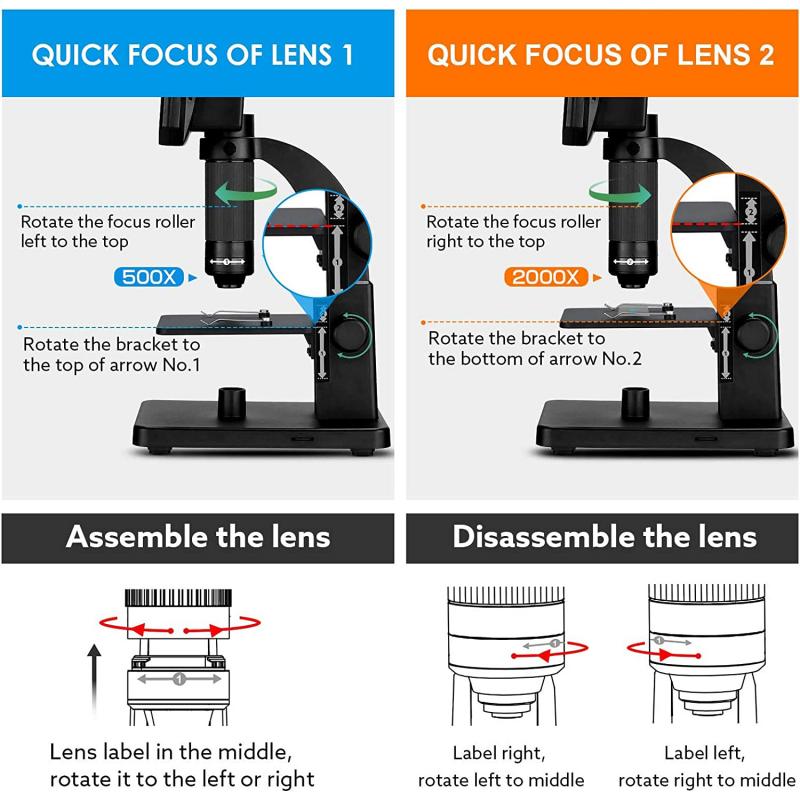
3、 Transmission Electron Microscopy: Indirectly observing atoms through electron scattering.
Can atoms be observed under a microscope? The answer is yes, but with some caveats. Traditional optical microscopes, which use visible light, have a limited resolution due to the wavelength of light. Atoms are much smaller than the wavelength of visible light, making them impossible to directly observe using this type of microscope.
However, with the advent of advanced microscopy techniques, such as Transmission Electron Microscopy (TEM), it is now possible to indirectly observe atoms. TEM uses a beam of electrons instead of light to image samples. Electrons have much shorter wavelengths than visible light, allowing for higher resolution imaging.
In TEM, a beam of electrons is transmitted through a thin sample, and the resulting image is formed by the interaction of the electrons with the atoms in the sample. By analyzing the pattern of electron scattering, scientists can infer the positions of atoms and obtain detailed information about their structure.
TEM has revolutionized our understanding of the atomic world. It has been used to observe individual atoms, study the arrangement of atoms in materials, and even visualize chemical reactions at the atomic scale. This technique has played a crucial role in fields such as materials science, nanotechnology, and biology.
It is important to note that while TEM allows us to indirectly observe atoms, it does not provide a direct visualization of individual atoms. The images obtained through TEM are representations of electron scattering patterns, and the interpretation of these patterns requires advanced analysis techniques.
In conclusion, while atoms cannot be observed directly under a traditional optical microscope, Transmission Electron Microscopy provides a powerful tool for indirectly observing atoms through electron scattering. This technique has greatly expanded our understanding of the atomic world and continues to push the boundaries of scientific discovery.

4、 X-ray Crystallography: Determining atomic positions by analyzing X-ray diffraction patterns.
Yes, atoms can be observed under a microscope using various techniques, including X-ray crystallography. X-ray crystallography is a powerful method for determining the positions of atoms in a crystal lattice by analyzing the diffraction patterns produced when X-rays interact with the crystal.
In X-ray crystallography, a crystal is bombarded with a beam of X-rays, which causes the X-rays to scatter in different directions. The scattered X-rays create a pattern of spots on a detector, which can be analyzed to determine the arrangement of atoms in the crystal. By measuring the angles and intensities of the diffracted X-rays, scientists can calculate the positions of the atoms within the crystal lattice.
X-ray crystallography has been widely used to determine the structures of a wide range of materials, including small organic molecules, inorganic compounds, and large biological macromolecules such as proteins and nucleic acids. It has provided valuable insights into the three-dimensional structures of these molecules, which are crucial for understanding their functions and designing drugs.
However, it is important to note that X-ray crystallography can only provide information about the positions of atoms in a crystal lattice. It cannot directly observe individual atoms or molecules in solution or in non-crystalline states. In recent years, advancements in techniques such as cryo-electron microscopy have allowed for the visualization of individual atoms and molecules in non-crystalline samples, providing complementary information to X-ray crystallography.
In conclusion, while X-ray crystallography is a powerful technique for determining atomic positions in crystals, it is not the only method available for observing atoms under a microscope. Other techniques, such as cryo-electron microscopy, have expanded our ability to visualize individual atoms and molecules in non-crystalline samples.