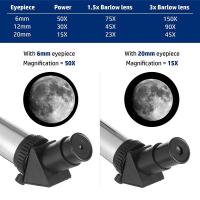
How Do Confocal Microscopes Work ?
Confocal microscopes work by using a pinhole aperture to eliminate out-of-focus light from the sample being imaged. A laser beam is used to illuminate the sample, and the light emitted from the sample is collected by a detector. The pinhole aperture is placed in front of the detector, and only light that is in focus with the aperture is allowed to pass through and be detected. This creates a sharp, high-resolution image of the sample.
The laser beam is scanned across the sample in a raster pattern, and the detector collects the emitted light at each point. The resulting data is used to create a 3D image of the sample, with each layer being in focus and free from the blur caused by out-of-focus light.
Confocal microscopes are commonly used in biological research, as they allow for high-resolution imaging of cells and tissues. They are also used in materials science and other fields where high-resolution imaging is important.
1、 Laser Scanning Confocal Microscopy
Laser Scanning Confocal Microscopy (LSCM) is a powerful imaging technique that allows for high-resolution, three-dimensional imaging of biological samples. The basic principle of LSCM is to use a laser to scan a focused beam of light across a sample, and then to collect the emitted fluorescence from the sample at each point of the scan. By collecting the fluorescence from only a small volume of the sample at a time, LSCM can produce images with high spatial resolution and excellent contrast.
The key components of an LSCM system include a laser, a scanning mechanism, a detector, and a computer for image processing. The laser is used to excite the fluorescent molecules in the sample, and the scanning mechanism is used to move the laser beam across the sample in a controlled manner. The detector collects the emitted fluorescence from the sample, and the computer processes the data to produce a three-dimensional image.
One of the major advantages of LSCM is its ability to produce images of thick samples with high spatial resolution. This is achieved by using a technique called optical sectioning, which allows for the collection of fluorescence from only a thin slice of the sample at a time. By scanning through the sample in this way, LSCM can produce a series of images that can be combined to create a three-dimensional image of the sample.
In recent years, LSCM has been used in a wide range of applications, including the study of cellular dynamics, the visualization of subcellular structures, and the analysis of tissue architecture. With continued advances in technology, LSCM is likely to remain an important tool for biological imaging in the years to come.
2、 Spinning Disk Confocal Microscopy
Spinning Disk Confocal Microscopy is a type of confocal microscopy that uses a spinning disk with multiple pinholes to create a series of optical sections through a sample. The spinning disk is placed in the path of the excitation light, which is focused onto the sample through a microscope objective. The pinholes in the disk allow only the light from the focal plane to pass through, while blocking out-of-focus light from above and below the plane. This creates a high-resolution image of the sample with improved contrast and reduced background noise.
The latest advancements in spinning disk confocal microscopy include the use of advanced imaging techniques such as fluorescence lifetime imaging (FLIM) and fluorescence correlation spectroscopy (FCS). FLIM allows for the measurement of the lifetime of fluorescent molecules, which can provide information about the local environment and interactions of the molecules. FCS measures the fluctuations in fluorescence intensity over time, which can provide information about the diffusion and binding of molecules in the sample.
Another recent development in spinning disk confocal microscopy is the use of super-resolution techniques such as structured illumination microscopy (SIM) and stochastic optical reconstruction microscopy (STORM). These techniques allow for the visualization of structures and molecules at a resolution beyond the diffraction limit of light, providing unprecedented detail and clarity in biological imaging.
Overall, spinning disk confocal microscopy is a powerful tool for high-resolution imaging of biological samples, and the latest advancements in the field continue to push the boundaries of what is possible in biological imaging.
3、 Multi-Photon Confocal Microscopy
Multi-Photon Confocal Microscopy is a type of confocal microscopy that uses longer wavelength light to excite fluorescent molecules in a sample. This technique allows for deeper imaging of thick samples, as the longer wavelength light can penetrate further into the sample without causing damage.
In a confocal microscope, a laser is used to illuminate a small point on the sample, and the emitted fluorescence is collected through a pinhole aperture. This pinhole aperture allows for the collection of only the light emitted from the focal plane, resulting in a sharper image with less background noise.
Multi-Photon Confocal Microscopy takes this a step further by using two or more photons to excite the fluorescent molecules. This allows for even deeper imaging, as the photons are absorbed by the sample in a non-linear manner, resulting in excitation only at the focal point.
Recent advancements in Multi-Photon Confocal Microscopy have allowed for even greater imaging depth and resolution. Adaptive optics can be used to correct for aberrations in the sample, resulting in sharper images. Additionally, new fluorescent probes have been developed that allow for imaging of specific cellular structures and processes.
Overall, Multi-Photon Confocal Microscopy is a powerful tool for imaging thick samples with high resolution and specificity. Its continued development and refinement will undoubtedly lead to even greater insights into the workings of biological systems.
4、 Super-Resolution Confocal Microscopy
Super-Resolution Confocal Microscopy is a technique used to obtain high-resolution images of biological samples. Confocal microscopes work by using a laser to illuminate a small area of the sample, and then detecting the light that is emitted from that area. The microscope then moves the laser to a different area of the sample and repeats the process, building up a 3D image of the sample.
Super-Resolution Confocal Microscopy takes this process a step further by using specialized techniques to improve the resolution of the images obtained. One such technique is called stimulated emission depletion (STED) microscopy, which uses a second laser to "deactivate" the fluorescent molecules in the sample, allowing for a sharper image to be obtained.
Another technique used in Super-Resolution Confocal Microscopy is called structured illumination microscopy (SIM), which uses a patterned light source to create interference patterns that can be used to improve the resolution of the image.
The latest point of view on Super-Resolution Confocal Microscopy is that it is a powerful tool for studying biological samples at the cellular and molecular level. It has been used to study a wide range of biological processes, including protein interactions, cell signaling, and the structure of cellular organelles. As technology continues to improve, it is likely that Super-Resolution Confocal Microscopy will become even more widely used in the field of biology.