How To Use Fluorescence Microscope ?
To use a fluorescence microscope, first, ensure that the microscope is properly set up and calibrated. Place the sample or specimen on the microscope stage and adjust the focus using the coarse and fine focus knobs. Next, select the appropriate fluorescence filters and insert them into the filter cube. Turn on the fluorescence light source and adjust the intensity as needed. Look through the eyepieces or use a camera to view the sample. Adjust the focus and other settings to obtain a clear and well-contrasted image. Finally, analyze and document the fluorescence signals using appropriate software or imaging techniques.
1、 Fluorescence microscopy: Principles and techniques
To use a fluorescence microscope, follow these steps:
1. Prepare your sample: Fix and stain your sample with a fluorescent dye or antibody that specifically binds to the target molecule you want to visualize. This can be done using various techniques such as immunofluorescence or fluorescent protein expression.
2. Set up the microscope: Turn on the microscope and adjust the light source to the appropriate wavelength for excitation of the fluorophore. Ensure that the microscope is properly aligned and focused.
3. Choose the appropriate filters: Select the appropriate excitation and emission filters for your fluorophore. These filters allow only the desired wavelengths of light to pass through, enhancing the contrast and specificity of the fluorescence signal.
4. Adjust the exposure settings: Adjust the exposure time and gain settings on the camera to optimize the signal-to-noise ratio. Be cautious not to overexpose the image, as this can lead to photobleaching and loss of fluorescence signal.
5. Capture images: Use the camera attached to the microscope to capture images of the fluorescently labeled sample. Take multiple images at different focal planes to ensure you capture the entire sample volume.
6. Analyze the images: Transfer the captured images to a computer and use image analysis software to process and analyze the data. This can include tasks such as quantifying fluorescence intensity, measuring colocalization, or tracking dynamic processes.
The latest advancements in fluorescence microscopy include the development of super-resolution techniques such as stimulated emission depletion (STED) microscopy and single-molecule localization microscopy (SMLM). These techniques allow for imaging beyond the diffraction limit, enabling the visualization of structures at the nanoscale. Additionally, advancements in fluorescent probes and dyes have improved the specificity and brightness of fluorophores, enhancing the sensitivity and resolution of fluorescence microscopy. Furthermore, the integration of fluorescence microscopy with other imaging modalities such as electron microscopy or light-sheet microscopy has enabled multimodal imaging, providing complementary information about the sample.

2、 Sample preparation for fluorescence microscopy
Sample preparation for fluorescence microscopy is a crucial step in obtaining accurate and reliable results. To effectively use a fluorescence microscope, it is essential to properly prepare the samples. Here is a step-by-step guide on how to prepare samples for fluorescence microscopy:
1. Fixation: Start by fixing the sample to preserve its structure and prevent degradation. Common fixatives include formaldehyde or paraformaldehyde. The fixation time may vary depending on the sample type and size.
2. Permeabilization: Some samples, such as cells, may require permeabilization to allow the fluorescent probes to enter. This step involves treating the sample with a permeabilizing agent like Triton X-100 or saponin.
3. Blocking: To reduce non-specific binding of fluorescent probes, it is crucial to block the sample. This is typically done by incubating the sample with a blocking agent such as bovine serum albumin (BSA) or non-fat dry milk.
4. Primary antibody incubation: If using immunofluorescence, incubate the sample with a primary antibody specific to the target of interest. The primary antibody will bind to the target protein or antigen.
5. Secondary antibody incubation: After washing away unbound primary antibodies, incubate the sample with a secondary antibody conjugated to a fluorophore. The secondary antibody will bind to the primary antibody, allowing visualization of the target.
6. Mounting: Finally, mount the sample onto a glass slide using an appropriate mounting medium. This will preserve the sample and prevent photobleaching.
It is important to note that sample preparation protocols may vary depending on the specific experiment and sample type. It is recommended to consult the latest literature and protocols specific to your research area for the most up-to-date techniques and reagents.
Additionally, advancements in sample preparation techniques, such as super-resolution microscopy and tissue clearing methods, have revolutionized fluorescence microscopy. These techniques allow for higher resolution imaging and deeper tissue penetration, enabling researchers to study complex biological processes with greater detail.

3、 Choosing the right fluorophores for your experiment
Choosing the right fluorophores for your experiment is crucial when using a fluorescence microscope. Fluorophores are molecules that emit light of a specific wavelength when excited by light of a different wavelength. They are used to label specific targets in biological samples, allowing researchers to visualize and study various cellular processes.
To select the appropriate fluorophores, several factors need to be considered. Firstly, the excitation and emission wavelengths of the fluorophores should match the capabilities of the fluorescence microscope being used. This ensures that the microscope can effectively excite and detect the emitted light. Additionally, the brightness and photostability of the fluorophores should be taken into account. Bright fluorophores produce a strong signal, while photostable fluorophores can withstand prolonged exposure to light without significant loss of signal.
Another important consideration is the compatibility of the fluorophores with the biological sample. Some fluorophores may have a higher affinity for certain cellular structures or molecules, allowing for more specific labeling. It is also important to consider any potential interactions between different fluorophores if multiple labels are being used simultaneously.
In recent years, there have been advancements in fluorophore technology that offer improved performance and versatility. For example, there are now fluorophores with enhanced brightness and photostability, allowing for longer imaging times and improved signal-to-noise ratios. Additionally, there are fluorophores that can be switched on and off using specific wavelengths of light, enabling precise control over fluorescence signals.
In conclusion, choosing the right fluorophores for your experiment is essential for successful fluorescence microscopy. Considering factors such as excitation and emission wavelengths, brightness, photostability, and compatibility with the biological sample will help ensure accurate and reliable results. Staying updated with the latest advancements in fluorophore technology can also enhance the quality and versatility of fluorescence microscopy experiments.
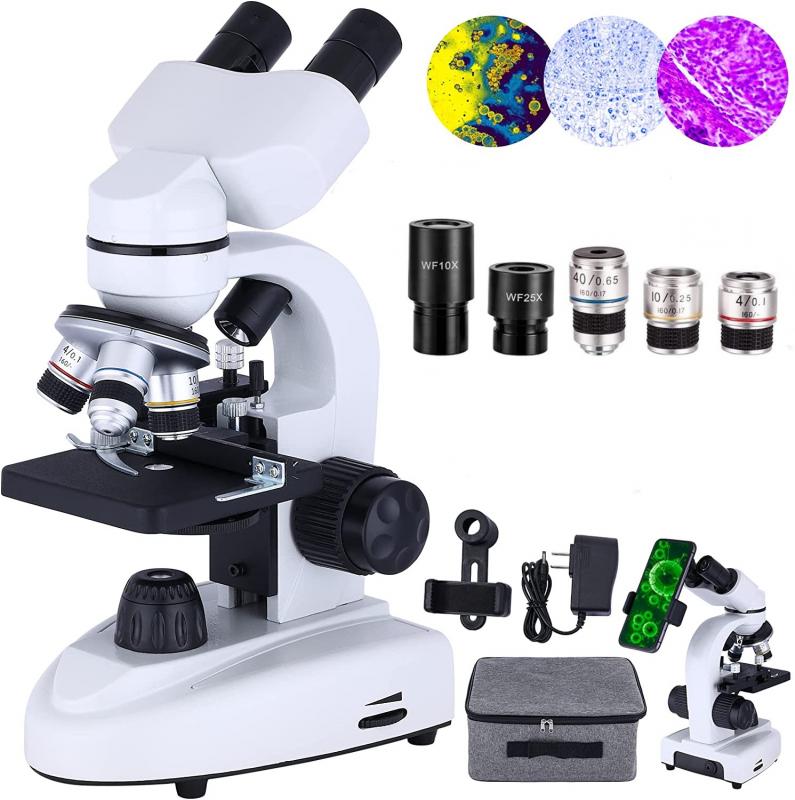
4、 Setting up the fluorescence microscope for optimal imaging
Setting up a fluorescence microscope for optimal imaging involves several steps to ensure accurate and high-quality results. Here is a guide on how to use a fluorescence microscope effectively:
1. Prepare the sample: Ensure that your sample is properly prepared for fluorescence imaging. This may involve fixing, staining, or labeling the sample with fluorescent dyes or proteins. Follow the specific protocols for your sample type to achieve the best results.
2. Choose the appropriate filters: Fluorescence microscopes use filters to select the excitation and emission wavelengths. Select filters that match the fluorophores used in your sample. This will allow you to selectively excite and detect the fluorescence signal.
3. Adjust the light source: Fluorescence microscopes typically use a mercury or LED light source. Adjust the intensity of the light source to an appropriate level for your sample. Avoid overexposure, as it can lead to photobleaching and phototoxicity.
4. Focus the sample: Use the appropriate objective lens and adjust the focus to obtain a clear image. Fluorescence imaging often requires a higher numerical aperture (NA) objective to capture fine details.
5. Set the exposure time: Determine the optimal exposure time for your sample. Longer exposure times can increase the signal-to-noise ratio but may also increase photobleaching. Experiment with different exposure times to find the balance between signal intensity and photostability.
6. Minimize background noise: To reduce background noise, use a darkroom or cover the microscope with a light-tight enclosure. This will help to eliminate stray light and improve the contrast of your fluorescence signal.
7. Capture and analyze the images: Use a camera or imaging software to capture the fluorescence images. Adjust the image settings, such as brightness and contrast, to enhance the visibility of the fluorescence signal. Analyze the images using appropriate software to quantify and interpret the data.
It is important to stay updated with the latest advancements in fluorescence microscopy techniques and technologies. New developments, such as super-resolution microscopy and advanced imaging modalities, can provide even higher resolution and more detailed information about cellular structures and processes.





























There are no comments for this blog.