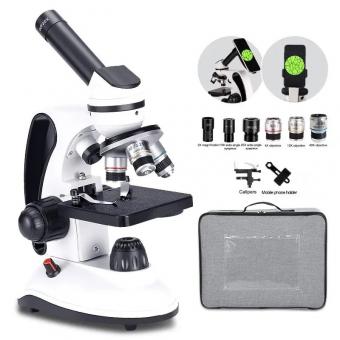
How To Prepare Specimen For Light Microscope ?
To prepare a specimen for a light microscope, start by selecting the sample you want to observe. Then, carefully place the sample on a glass slide. If the sample is too thick, you may need to slice it into thinner sections using a sharp blade or a microtome. Next, add a drop of a suitable mounting medium, such as water or a specialized mounting solution, to the slide. This helps to preserve the sample and prevent it from drying out.
Once the mounting medium is added, gently lower a coverslip onto the sample. Be careful to avoid trapping air bubbles between the slide and the coverslip. If necessary, you can use a fine needle or a pair of forceps to carefully remove any air bubbles.
Finally, clean the slide and coverslip to remove any excess mounting medium or debris. You can use a lens tissue or a cotton swab moistened with lens cleaning solution for this purpose. Once the slide is clean, it is ready to be placed on the stage of the light microscope for observation.
1、 Fixation: Preserving the structure and preventing decay.
To prepare a specimen for light microscopy, the first step is fixation. Fixation involves preserving the structure of the specimen and preventing decay. This is crucial as it allows for accurate observation and analysis under the light microscope.
There are various fixation methods available, and the choice depends on the type of specimen and the purpose of the study. Common fixatives include formaldehyde, glutaraldehyde, and ethanol. These fixatives work by cross-linking proteins and other cellular components, stabilizing the structure and preventing degradation.
The fixation process typically involves immersing the specimen in the fixative solution for a specific period of time. The duration of fixation depends on the size and type of the specimen, as well as the fixative used. It is important to follow the recommended fixation times to avoid over-fixation, which can lead to artifacts and distortion of the specimen.
In recent years, there has been a growing interest in alternative fixation methods that aim to preserve the native structure of the specimen. These methods, such as cryofixation and chemical fixation with less harsh fixatives, allow for better preservation of cellular components and molecular structures. Cryofixation involves rapidly freezing the specimen to extremely low temperatures, preserving the specimen in a near-native state. Chemical fixation with less harsh fixatives, such as paraformaldehyde, can also provide better preservation of delicate structures.
After fixation, the specimen is typically dehydrated using a series of ethanol or acetone washes. This removes water from the specimen, preparing it for embedding in a solid medium, such as paraffin or resin. The embedded specimen is then sliced into thin sections using a microtome and mounted onto glass slides.
In conclusion, fixation is a crucial step in preparing a specimen for light microscopy. It preserves the structure of the specimen and prevents decay, allowing for accurate observation and analysis. The choice of fixative and fixation method depends on the type of specimen and the purpose of the study. Recent advancements in fixation techniques aim to preserve the native structure of the specimen, providing better insights into cellular and molecular processes.

2、 Dehydration: Removing water to prevent distortion during staining.
To prepare a specimen for a light microscope, one important step is dehydration. Dehydration involves removing water from the specimen to prevent distortion during staining. This process is crucial as water can cause cells to swell and distort, making it difficult to observe the specimen accurately under a microscope.
There are several methods to dehydrate a specimen. One common technique is the use of alcohol series, where the specimen is gradually immersed in a series of alcohol solutions with increasing concentrations. This allows for the gradual removal of water from the specimen while preserving its structure. The alcohol displaces the water in the cells, preventing distortion.
Another method is freeze-drying, also known as lyophilization. This technique involves freezing the specimen and then subjecting it to a vacuum, which causes the frozen water to sublimate directly from solid to gas, bypassing the liquid phase. This method is particularly useful for delicate specimens that may be damaged by the dehydration process.
It is important to note that while dehydration is a necessary step in preparing a specimen for a light microscope, it can also lead to some limitations. Dehydration can cause shrinkage of the specimen, which may affect the accuracy of measurements and the overall representation of the specimen. Additionally, some cellular components may be lost during the dehydration process, leading to potential loss of information.
In recent years, there has been a growing interest in alternative methods to minimize the distortion caused by dehydration. One such approach is the use of cryopreservation, where the specimen is rapidly frozen to preserve its structure without the need for dehydration. This technique has shown promising results in maintaining the integrity of the specimen and reducing distortion.
In conclusion, dehydration is an essential step in preparing a specimen for a light microscope. It helps prevent distortion during staining by removing water from the specimen. However, it is important to consider the limitations of dehydration and explore alternative methods, such as cryopreservation, to minimize distortion and preserve the integrity of the specimen.

3、 Clearing: Replacing dehydrating agent with a transparent medium.
To prepare a specimen for a light microscope, one important step is clearing, which involves replacing the dehydrating agent with a transparent medium. Clearing is necessary to enhance the visibility of the specimen and allow light to pass through it more easily, enabling better observation under the microscope.
The process of clearing begins after dehydration, where the specimen is gradually dehydrated using a series of alcohol solutions. Once dehydration is complete, the dehydrating agent, typically alcohol, needs to be replaced with a transparent medium. This is done to prevent the specimen from becoming opaque and to improve the refractive index, allowing light to pass through the specimen more effectively.
There are various clearing agents available, such as xylene, benzene, and cedarwood oil. These agents have different refractive indices and viscosities, which can affect the clearing process and the quality of the final image. Xylene is commonly used as a clearing agent due to its high refractive index and low viscosity, making it suitable for most specimens. However, it is important to note that xylene is toxic and should be handled with caution.
In recent years, there has been a growing interest in using alternative clearing methods, such as the CLARITY technique. CLARITY involves removing lipids from the specimen and replacing them with a hydrogel matrix, which renders the tissue transparent. This technique allows for the visualization of intact structures within the specimen, providing a three-dimensional view under the microscope.
In conclusion, clearing is an essential step in preparing a specimen for a light microscope. By replacing the dehydrating agent with a transparent medium, the visibility of the specimen is enhanced, enabling better observation and analysis. The choice of clearing agent depends on the nature of the specimen and the desired imaging outcome. Additionally, emerging techniques like CLARITY offer new possibilities for transparent specimen preparation, allowing for more detailed and comprehensive analysis.

4、 Embedding: Encasing specimen in a solid medium for sectioning.
To prepare a specimen for light microscopy, one common method is embedding, which involves encasing the specimen in a solid medium for sectioning. This process allows for thin slices of the specimen to be obtained, which can then be mounted on a microscope slide for observation under a light microscope.
The first step in embedding is fixation, where the specimen is treated with a fixative solution to preserve its structure and prevent decay. Common fixatives include formaldehyde or glutaraldehyde, which crosslink proteins and stabilize cellular components. After fixation, the specimen is dehydrated using a series of alcohol solutions to remove water and replace it with a solvent that is compatible with the embedding medium.
Next, the specimen is infiltrated with an embedding medium, such as paraffin wax or plastic resin. Paraffin wax is commonly used for routine histology, while plastic resins like epoxy or acrylic are used for more specialized applications. The specimen is immersed in the melted embedding medium, which infiltrates the tissue and replaces the solvent. The medium is then allowed to solidify, encasing the specimen.
Once the embedding medium has solidified, the specimen is ready for sectioning. Thin slices, typically around 5-10 micrometers thick, are cut using a microtome. These sections are then mounted on microscope slides and stained to enhance contrast and visualize specific structures or components of interest.
It is worth noting that with advancements in microscopy techniques, alternative methods to traditional embedding have emerged. For example, cryosectioning involves freezing the specimen without the need for embedding, allowing for rapid sectioning of fresh or frozen samples. Additionally, techniques like optical clearing and 3D imaging have revolutionized the field by enabling the visualization of intact, transparent specimens without the need for sectioning.
In conclusion, embedding is a widely used method for preparing specimens for light microscopy. It involves fixation, dehydration, infiltration with an embedding medium, sectioning, and staining. However, with the continuous development of new techniques, researchers now have a range of options to choose from based on the specific requirements of their study.